Styrenic Block Copolymers (SBCs) are the largest-volume category of thermoplastic elastomers. Annual consumption is about 1.200.000 metric ton. Being thermoplastic elastomers, SBCs possess the mechanical properties of rubbers and the processing characteristics of thermoplastics. This is related to their molecular structure. SBCs consist of at least three blocks, namely two hard polystyrene end blocks and one soft, elastomeric (polybutadiene, polyisoprene, either or not hydrogenated) midblock. It is essential that the hard and soft blocks are immiscible, so that, on a microscopic scale, the polystyrene blocks form separate domains in the rubber matrix, thereby providing physical cross-links to the rubber.
Upon raising the temperature above the Tg (± 100 o C) of polystyrene or on bringing the material into a hydrocarbon solvent, the polystyrene domains disintegrate and the SBCs become processable as thermoplastics. When solidified, SBCs exhibit good elastomeric qualities. Tensile strength is higher than for unreinforced vulcanized rubbers. Elongation at break ranges from 500 % to 1200 % and resilience is comparable to that of vulcanized rubbers. Melt viscosity is comparable to that of thermoplastics, such as polystyrene and polypropylene.
Discovered and commercialized in the early sixties, SBCs have since found numerous applications. Their typical balance between properties and processability leads to focusing on unique applications instead of replacing the general-purpose rubber.
SBCs can be readily mixed with other polymers, oil, and fillers, which allows versatile tuning of product properties. They are employed in enhancing the performance of bitumen in road paving and roofing applications, particularly under extreme weather conditions. They are widely applied in adhesives, sealants, coatings, and in footwear. Also, SBCs are compounded to produce materials that enhance grip, feel, and appearance in applications such as toys, automotive, personal hygiene, and packaging.
Styrenic Block Copolymers are made by anionic polymerization with an organometallic catalyst, such as butyllithium, as an initiator. The polymerization concerns a living polymerization and generally takes place in batch in a non-polar hydrocarbon solvent. Thermal die out is limited as long as the maximum polymerization temperature does not exceed 80° C.
Chemicaldie out is limited by rigorous purification of the materials used. The molecular weight is well defined and can be predicted. The resulting narrow molecular-weight distribution (Polydispersity Index: 1.04 – 1.05) is essential for the phase separation that is responsible for the elastomeric properties. Typical molecular weights range from 100.000 to 300.000 g/mole.
In non-polar media and for typical initiator concentrations and polymer molecular weights, the microstructure of the SBC midblock is about as follows. The butadiene midblock consists of 35 % cis-1,4 , 55 % trans-1,4 ,and 9 % 1,2 (vinyl) insertion, the isoprene midblock of 70 % cis-1,4 , 25 % trans-1,4 , and 5 % 1,2 or 3,4 (vinyl) insertion. Upon adding polar components to the reaction medium, the ratio of vinyl insertion increases.
Polymerization can be carried out sequentially: after polymerizing the first polystyrene block, butadiene or isoprene is added, followed by polymerization of the polystyrene end block. Another approach consists of polymerization of polystyrene poly-diene diblock, followed by coupling two (or more) living di-blocks by a di- (or multi-) functional coupling agent. This allows the synthesis of multiblock radial SBCs.
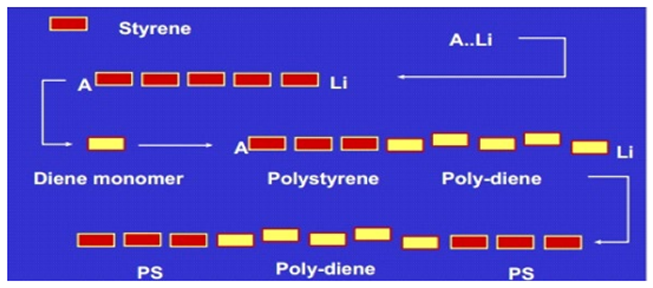
Because of the presence of double bonds in the poly-diene mid-blocks, both SBS and SIS are vulnerable to thermal and oxidative degradation. For polybutadiene, degradation generally occurs through cross-linking, for polyisoprene through chain scission. By selectively hydrogenating the midblock, SBCs become substantially more stable.
However, when standard polybutadiene with only 9 % 1,2-insertion is hydrogenated, the poly-ethylene-like material is obtained, which results in crystallization of the midblock and a concomitant loss of rubber properties. To avoid that happening, polybutadiene is polymerized in the presence of a modifier, resulting in a high 1,2- insertion. The resulting hydrogenated midblock then is ethylene-butene (EB), the copolymer is SEBS.
In Figure 1 below a typical manufacturing scheme for the anionic polymerization of SBCs is presented. The process starts with rigorous feed treatment, in which polar impurities (a.o. water) have to be removed from all feed used.
Also, the monomer feedstocks have to be cleaned from polymerization inhibitor. Purification techniques used include distillation and the use of activated absorbents such as alumina and molecular sieves.
In general anionic polymerization is a batch process in solution. The heat of polymerization is considerable and a variety of heat removal techniques are applied,
such as jacket cooling, coil cooling, pump-around cooling via an external loop, and evaporative (reflux) cooling. Because of thermal die out, the highest applicable polymerization temperature is approximately 80° C. The reaction can be carried out isothermally or (partially) adiabatically. After the polymerization, a blending step acts as a buffer between the batch polymerization and the continuous work up. Besides, blending ensures smoothening of batch-to-batch variation.
There are two different solvent removal processes. The first method concerns direct desolventizing in a (vacuum) extruder, where the solvent is evaporated. This results in a rubber melt, which is pressed through a die-plate into pellets and cooled. The second approach concerns steam stripping (steam coagulation) in which the SBC solution is contacted with an excess of steam and water. The solvent evaporates and the result is a mixture of rubber crumbs in water. Subsequently, water removal takes place either by a dewatering extruder followed by a hot air dryer or by expeller-expander technology. The latter consists of a dewatering extruder followed by a second extruder where the remaining water is superheated and evaporates instantly at the die-plate, thereby expanding and rupturing the rubber into an open crumb structure. The finishing process aims at end products that are virtually water- and solvent-free. The appearance of the end product depends on the exact solvent removal and drying technologies employed. Both dense pellets and porous pellets are available. Other product morphologies are open crumbs and milled powder.
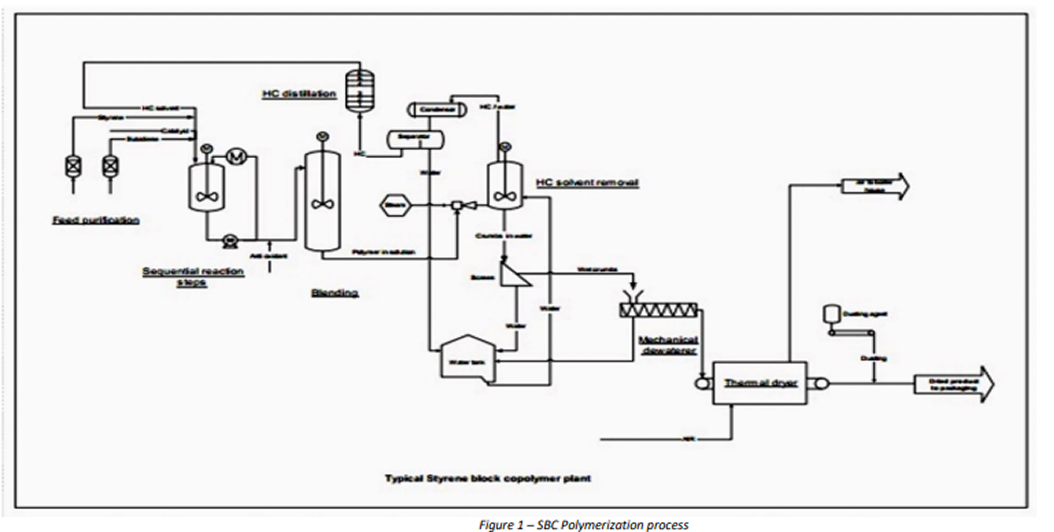
In case of a product containing hydrogenated midblock, a hydrogenation step is inserted after the blending of polymerization product. Subsequently, the hydrogenation catalyst is washed out, after which the solvent is removed and the product is dried.
Styrenic Block Copolymers are high-performance thermoplastic elastomers engineered to enhance the performance capabilities of a wide spectrum of end products and applications. Its versatility in compounding enables extensive tuning of product properties, which allows the market to keep growing into new directions.
Building a Sutainable Future for Your Organization and the Synthetic Rubber Industry
Download here

Address:
16360 Park Ten Place Suite 110
Houston, TX 77084
Contact Us:
+1 713 783 1703
info@iisrp.com