General-purpose grades are mostly produced with n-dodecyl mercaptan as the chain transfer agent and occasionally with xanthogen disulfides. If xanthogen disulfides are used, the elastomers are more readily processable and give vulcanizates with improved mechanical properties.
Pre crosslinked grades consist of a blend of soluble polychloroprene and crosslinked polychloroprene. They show less swelling after extrusion (die swell) and better calender ability. Pre crosslinked grades are particularly suitable for the extrusion of profiled parts.
Sulfur-modified grades are copolymers of chloroprene and elemental sulfur. The viscosity is adjusted – in contrast to general-purpose grades - mostly after polymerization by “peptization” of the polysulfide bonds by e.g. thiuram disulfides as peptization agents. Sulfur modification improves the breakdown of the rubber during mastication (lowering of viscosity). Sulfur-modified grades are used in particular for parts exposed to dynamic stress, such as drive belts, timing belts or conveyor belts because of their excellent mechanical properties. But the polymers are less stable during storage and the vulcanizates less resistant to aging.
Slow crystallizing grades are polymerized with 2,3-dichloro-1,3-butadiene as a comonomer. This comonomer reduces the degree of crystallization by introducing irregularities into the polymer chain. High polymerization temperatures also make structural irregularities, if this comonomer is not available. Crystallization resistant grades are used to produce rubber articles, which have to retain their rubbery properties at very low temperatures.
Polychloroprene will continue to be one of the most important synthetic specialty elastomers because its balance of properties is unique.
Modern synthetic polyisoprene is designed to be similar to natural rubber in structure and properties. Although it still demonstrates lower green strength, slower cure rates, lower hot tear, and lower aged properties than its natural counterpart, synthetic polyisoprene exceeds the natural types inconsistency of product, cure rate, processing, and purity. In addition, it is superior in mixing, extrusion, molding, and calendering processes.
The successful synthesis of stereoregular polyisoprene (IR) fulfilled a goal sought by polymer chemists for nearly a century. Researchers knew that isoprene was the building block for natural rubber, and through the years, many attempts were made to synthesize materials with similar properties. Initially, the resulting polymers failed to exhibit some of the desired aspects of natural rubber because of differences in microstructure, which plays an important role in polyisoprene’s physical properties.
The polymer chains in the early synthetics contained mixtures of all possible molecular configurations joined together in a random fashion. Specifically, they lacked the very high cis-1,4 structure of the natural rubber backbone that gives it the ability to undergo strain crystallization.
Initial commercialization of a stereoregular, low cis-1,4 IR (90% to 92%) was realized in 1960 by Shell Chemical Company with the introduction of Shell Isoprene Rubber, produced with an alkyl lithium catalyst (Li-IR). However, the cis-1,4 content of Li-IR was insufficient to achieve the important crystallization properties of natural rubber.
In 1962, Goodyear introduced NATSYN®, a Ziegler-Natta (titanium-aluminum) catalyzed IR (Ti-IR) with a cis-1,4 content of 98.5%, finally allowing the benefits of straincrystallization to be realized. Goodrich-Gulf introduced another Ti-IR polymer about three years later but subsequently withdrew from the market in 1978. The manufacture of high cis IR has since been undertaken elsewhere, primarily in Russia and Japan.
In the mid-1950s, researchers discovered and developed new types of catalyst systems that could selectively join together monomer units in a well-ordered fashion. Shortly after Karl Ziegler’s breakthroughs in catalyst systems for the polymerization of ethylene, similar catalysts were developed for use with isoprene. These “stereospecific” catalysts allowed realization of a nearly pure cis-1,4 structure, and in doing so, the production of a synthetic natural rubber.
In addition to the cis-1,4 configuration, several other IR microstructures have been reported. A high trans-1,4 structure was produced by Polysar and is now being produced by Kuraray. This polymer has significant room temperature crystallinity and is a synthetic analog of the naturally occurring Balata. A Li-IR with increased 3,4 structure can be prepared by adding polar modifiers to the alkyl lithium catalyst system. However, since the higher cis-1,4 configuration most closely mirrors the properties of natural rubber and is the most important commercially, it will be the focus of this article.
Currently, synthetic polyisoprene is being used in a wide variety of industries in applications requiring low water swell, high gum tensile strength, and good resilience, high hot tensile and good tack. Gum compounds based on synthetic polyisoprene are being used in rubber bands, cut thread, baby bottle nipples, and extruded hose. Black loaded compounds find use in tires, motor mounts, pipe gaskets, shock absorber bushings and many other molded and mechanical goods. Mineral-filled systems find applications in footwear, sponge, and sporting goods. In addition, recent concerns about allergic reactions to proteins present in natural rubber have prompted increased usage of the purer synthetic polyisoprene in some applications.
Consumption of synthetic polyisoprene stabilized in the early 1990’s as polyisoprene’s availability was limited by manufacturing capacity and monomer availability. Recent increases in capacity, concerns about the stability of the price of natural rubber, and the mandate to move away from natural rubber in certain applications provide avenues for future growth in the industry. The table below shows the world consumption history & forecast consumption (years 2012-2015) of synthetic polyisoprene.
Typical raw polymer and vulcanized properties of polyisoprene are similar to values obtained for natural rubber. Natural rubber and synthetic polyisoprene both exhibit good inherent tack, high compounded gum tensile, good hysteresis, and good hot tensile properties.
The very specific nature of synthetic polyisoprene provides a number of factors that differentiate it from natural rubber. There is minimal variance in physical properties lot to lot. Polymerization conditions are narrowly controlled to assure that the polymer is highly specific chemically. There is a low level of non-polymer constituents as compared to natural rubber.
Synthetic polyisoprene’s ease of processability is of importance where consistency and quality are major considerations. Since less mechanical work and breakdown are required, shorter mix cycles and the elimination of pre-massing are possible when it is used as a direct replacement for natural rubber. The end results are time and power savings as well as increased throughput. In addition, synthetic polyisoprene exhibits greater compatibility than natural rubber in blends with solution SBR and EPDM.
Examples of Consumer Products Which Contain Polyisoprene:
Synthetic polyisoprene’s uniformity is a factor where the desire for consistent quality is paramount, as is increasingly the case in many industries with an emphasis on precise dimensional control in processing.
Because of the lower raw polymer viscosity of synthetic polyisoprene, part or the entire breakdown step normally used for natural rubber should be eliminated. Synthetic polyisoprene compounds at the same plasticity of natural rubber will have less die swell because of having less nerve. Also, at the same plasticity, the synthetic polymer will have significantly faster extrusion rates.
Synthetic polyisoprene compounds can be adapted for curing in any conventional molding operation whether it is compression, transfer, or injection. Synthetic polyisoprene is especially well suited for injection molded compounds. Because of its uniform cure rate, exact time/temperature press cycles can be established with the assurance that all pieces will be uniformly cured. In addition, the Mooney of synthetic polyisoprene reduces injection pressures and times with a resultant increase in output.
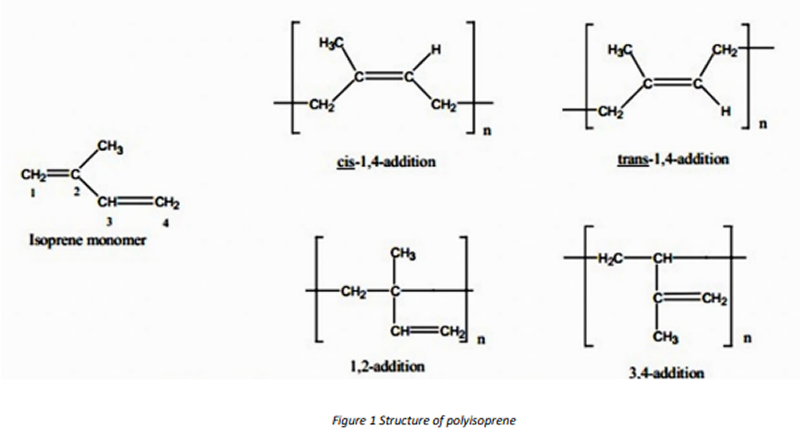
From the preceding discussion, it can be seen that many chemical structures (microstructures) are possible and that different catalyst systems result in specific microstructures with different physical properties.
The polymerization of isoprene monomer can proceed in a 1,2-, 3,4-, or 1,4- mode to give the structures shown in Figure 12. The structural content of natural rubber, TiIR and Li-IR are given in Table 1.
| NR | Ti-IR | Li-IR | |
|---|---|---|---|
| Microstructure | |||
| Cis-1,4 | 100 | 98.5 | 90 |
| Trans-1,4 | 0 | 1 | 5 |
| 3,4 | 0 | 0.5 | 5 |
| Purity | |||
| Rubber content | 94 | >99.0 | >99.0 |
Stress crystallization in cis-1,4 IR leads to important physical properties such as green strength, tear strength, and gum tensile strength. Research has shown that there are major differences in the ability of the cis-1,4-IRs to crystallize depending on the level and nature of the cis microstructure found in the polymer. For Li-IR, while x-ray diffraction patterns have indicated some crystallinity in stretched specimens, no crystallinity is seen in the unstretched state.
Ti-IR and NR both undergo crystallization in the unstretched state at low temperatures (the maximum rate of crystallization occurs at –25o C), but the rate is greatest for NR.
Both undergo crystallization at room temperature on stretching, with NR stress crystallizing at a lower elongation than Ti-IR.
Figure 2 depicts a simplified flow diagram for an isoprene polymerization process. Before entering the reactors, the solvent, catalyst, and isoprene monomer must be free of chemical impurities, moisture, and air—all of which are catalyst poisons. The purified streams first enter a chain of reactors in series into which the catalyst is injected, and the polymerization begins.
After the desired extent of polymerization has been attained, a shortstop or catalyst deactivator is added to the cement so no further linkage of monomer or polymer takes place.
In the next step, the cement mixture is put through a stripping operation whereby the solvent is recovered and the polymer cement converted to a crumb by hot water and steam. The crumb slurry is processed through extruders to remove water before it is cooled, baled, packaged, and placed in storage ready for shipment.
A non-staining antioxidant is then added to protect the polymer during finishing and storage.
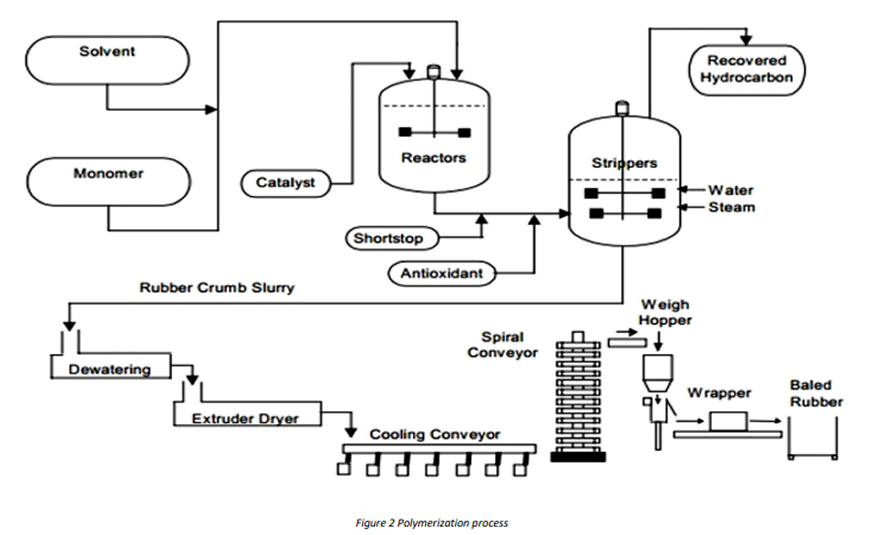
Synthetic polyisoprene represents one of the important classes of polymers produced in a solution medium. The successful development of a stereospecific catalyst system has permitted the production of a synthetic analog of natural rubber with improved uniformity and processing.
Building a Sutainable Future for Your Organization and the Synthetic Rubber Industry
Download here

Address:
16360 Park Ten Place Suite 110
Houston, TX 77084
Contact Us:
+1 713 783 1703
info@iisrp.com