Styrene - Butadiene Rubber also referred to as SBR, is a random copolymer of styrene and butadiene. There are two major types of SBR, Emulsion-SBR (E-SBR) and Solution-SBR (S-SBR), based on the different manufacturing process.
The unique nature of the insertion of butadiene on the growing chain, particularly butadiene- 1,4 and 1,2-additions, as well as the formation of the two cis-1,4 and trans-1,4- stereoisomers, implies the presence of four monomer units comprising a copolymer.
This remark acquires a particular meaning if we consider the physical and rheological characteristics of the finished polymer. The ratio of the structural unit content of styrene, and of butadiene inserted in 1,4- and 1,2-addition mode along the chain is the most important parameter influencing the glass transition temperature (Tg) of the material.
Interestingly, the concentration of 1,4-trans units has a strong influence on the strain induced crystallization of the rubber, leading to an improved rubber reinforcement and to increased tensile ultimate properties. Interestingly, natural rubber usually referred to as high cis-polyisoprene shows a strain-induced crystallization behavior as well.
Moreover, the relative concentration of 1,4- and 1,2-units may influence the thermal stability of the polymer. The oxidative degradation of the rubber starts from the addition of oxygen to a double bond: if the double bond is located in the main chain, such as in the case of 1,4 units, the reaction leads to a chain scission.
In recent years, because of the interest in fuel saving, developmental efforts led to new tire rubbers showing reduced dissipation of energy, corresponding to a lower rolling resistance, higher traction during braking and higher wear resistance (low abrasion).
Currently, most relevant trends in terms of SBR development (mainly focused on Solution-SBRs, due to their hysteresis properties in cured rubber formulations compared with Emulsion SBR based vulcanizates) for tire applications are
• Modification of the macrostructure (ie width of the Molecular Weight Distribution (MWD); presence, concentration, and distribution of Long Chain Branching (LCB)) and of the microstructure (ie monomer composition ratio, control of the addition of 1,2 butadiene units (vinyl)
• The preparation of the compound, searching for the best possible compromise between the quality of mixing and the mixing time. In this sense, the flow characteristics of the rubber are the high importance and the macrostructural features are the practical tools by means of which the rheological parameters are controlled (ie pseudo-plasticity, extensional viscosity, swelling, etc.). Furthermore, the interaction with the filler plays an important role. In addition, the search for additives/modifiers (e.g. silanes) that can be added to SBR at a non-cured compound stage to improve properties needs to be mentioned.
• Research on suitable polymer functionalization, to achieve specific chemical interactions with fillers, such as carbon black and silica. In addition, functionalized rubber may show an improved adhesion behavior onto certain tire components, namely onto the steel cords of the tire carcass.In both cases, the goal is to improve the rheological behavior of the polymer in all the various phases of its “service life”, namely:
• The ease of processing, which could be defined as the easiness for a certain material, to be melted, conveyed and shaped even into complex items, without an excessive consumption of energy and time and without any significant change in its final characteristics (ie degradation). The vulcanization process is crucial in SBR manufacturing and must be considered as part of the overall processing cycle
• The end-use behavior of the manufactured. In case of tires and, in particular, of the treads, “solid state properties” are discussed. In this sense, the most important characteristics are the dynamic properties which exist in different service situations, the mechanical characteristics (ie tensile strength, elongation at break) and the resistance to abrasion. The polymer microstructure (which determines the Tg), together with the homogeneity and quality of the vulcanized compound, influences the dynamic properties and the resistance to abrasion, while the mechanical behavior is strongly related to the polymer macrostructure (molecular weight distribution - MWD, long chain branching - LCB) and to the final result of the vulcanization.
It would be correct to add the efforts to optimize S-SBR production including: (i) increasing the monomer / solvent ratio in order to reduce the cost of the solvent recovery; (ii) treatment and to cut the emission of low molecular weight hydrocarbons (ie n-hexane, cyclohexane, traces of the residual monomers) into the environment.
One solution for the aforementioned problems may be (solvent-free) mass polymerization of styrene and butadiene, based on anionic initiators. A processing machine like an extruder (or similar machinery) appears to be an answer to problems which are associated with mass polymerization, including run-away reactions, the conveying of fluids, particularly dramatically varying viscosities during polymerization and the recovery or residual monomers.
E-SBR and S-SBR grades compete, but usually, S-SBR grades are applied to high-performance tire applications. While beneficially standardized ESBR grades supplied by several producers can be relatively exchanged, while the replacement of ESBR by SSBR for high-performance tires requires tire compound re-formulation and often new processing equipment, and thus significant investment.
So, one of the main efforts of the S-SBR producers has been the search for a better processability, which is achieved through modification of the polymer macrostructure (ie MWD and LCB). With this respect, the use of coupling agents, such as for example SiCl4 and SnCl4, primarily in batch-wise anionic polymerization provided an option to broaden the molecular weight distribution (MWD) and to form star-shaped polymer macromolecules.
Automotive companies have continuously developed vehicles with superior performance and durability (increased mileage). This has resulted in increasingly stringent tire performance specifications. These have been partly met by redesigning the structure components (ie tread patterns and reformulating compounds).
Due to their low resistance, high grip and abrasion resistance properties in tires, tire manufacturers have found no alternative to S-SBR grades for highly specified, high-performance tire components. The need for reducing fuel consumption led to the definition of rolling resistance specifications of the tire tread material. In the U.S. the CAFE (Corporate Average Fuel Economy) regulations are increasingly demanding high fuel efficiency and excellent grip & wear properties. Aforementioned performances specifications cannot be achieved with E-SBR, boosting the marked extension on S-SBR.
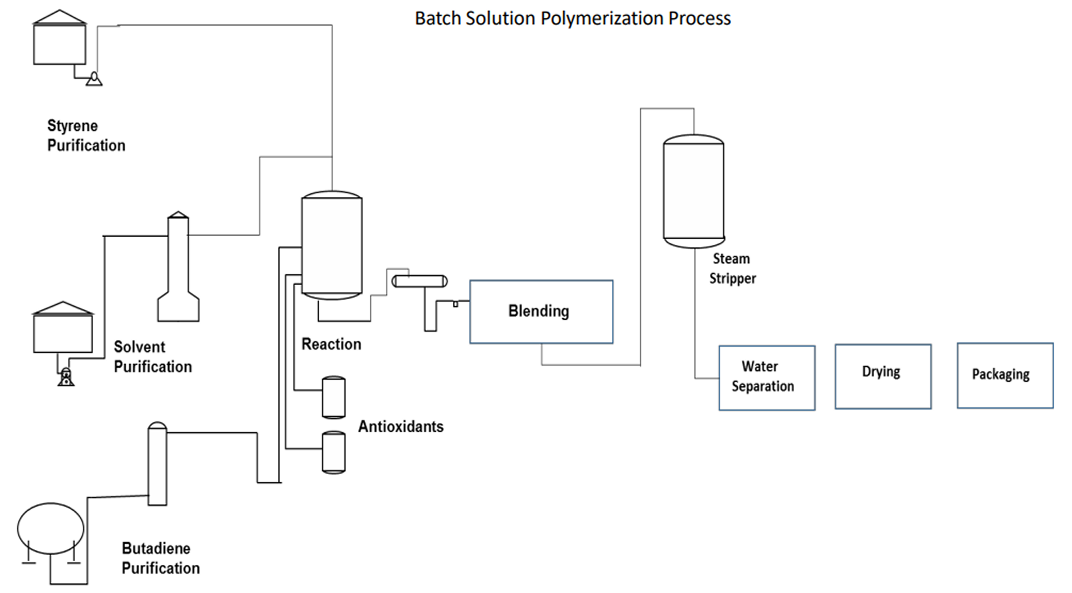
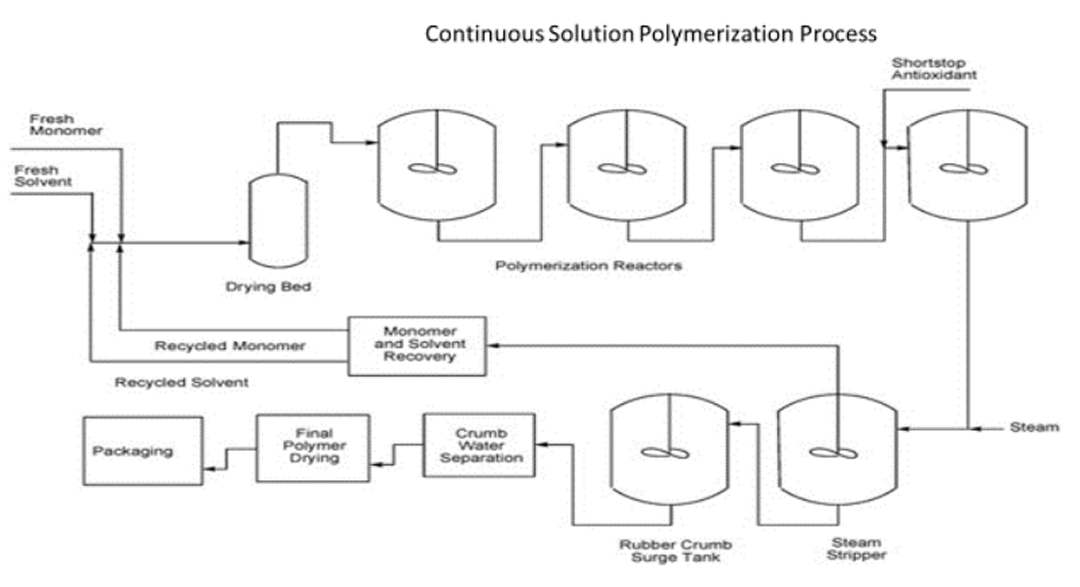
The production of emulsion and solution SBR can be carried out by means of two process configurations, batch and continuous process configuration. Typically, the E SBR process is continuous, while S-SBR facilities exist which are based on the continuous and batch operation.
The batch process allows a better and easier “fine-tuning” of the reaction conditions and, therefore, of the polymer characteristics. Solution SBR is made through anionic “living” polymerization usually initiated by alkyl lithium compounds, especially n- or sec.-butyl lithium. Alkyl lithium compounds are selected (a) due to their solubility in many solvents including technical relevant hydrocarbon solvents, such as n-hexane and cyclohexane, and (b) due to their relatively high base strength which is required to initiate polymerization.
Quantitative monomer conversion can be considered a beneficial feature of a “living” anionic solution polymerization. Accordingly, there is usually no need to isolate and recycle residual monomer which remained in the polymerization solvent upon termination of the polymerization process. In contrast, technical emulsion polymerization production processes require monomer recovery. Reportedly only about 70 % of the monomers are currently converted into polymers, including oil comprising ESBR versions.
There is a broad range of possible S-SBR micro- and macro structures. Therefore, only a more generalized procedure for SSBR production is presented below. The procedure refers to a copolymer comprising 25% styrene, while the vinyl content may vary from 10 to 60% depending on the kind and concentration of the randomizing Lewis acid (also called randomized agent).
Building a Sutainable Future for Your Organization and the Synthetic Rubber Industry
Download here

Address:
16360 Park Ten Place Suite 110
Houston, TX 77084
Contact Us:
+1 713 783 1703
info@iisrp.com